Project
Development of a CDISC-based Integrated Clinical trial system
Project Summary
- Cloud-Based eCRF Support (CRDMata)
- Clinical trial integrated management system services (CRD PMS)
- CDISC Clinical trial data system
- Clinical trial integrated management system services (CRD PMS)
- CDISC Clinical trial data system
Business Model
CDISC-based International Standard Clinical trial data solution
Main Achievements
2017.10 ~ 2017.11 Completion of IP NARAE Business
2017.11 Start-up / Scale up Selection
2017.11 CDISC Gold Member
2017.12 Completion of International intellectual property right-related dispute Prevention Consulting Business
2017.12 Venture business authorization
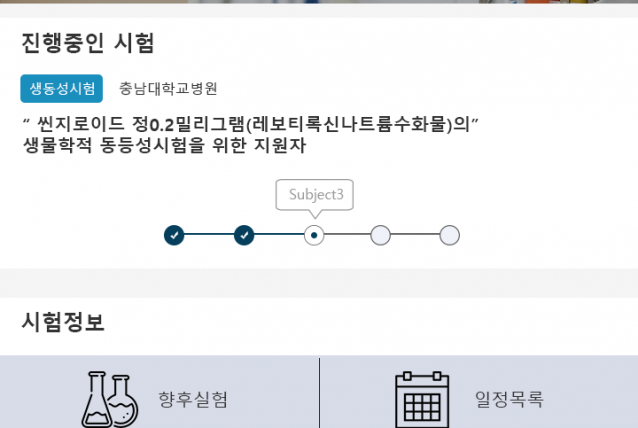
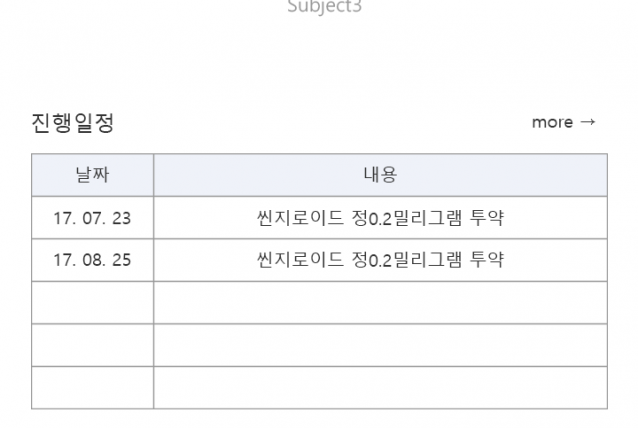
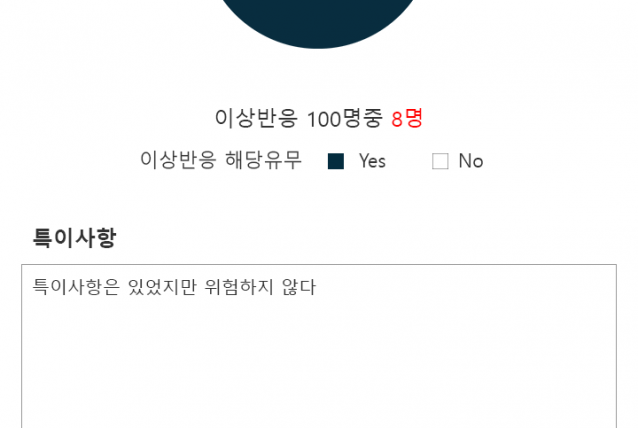
2017.07~ 2018.01 Clinical trial performance (Seven Bioquivalence Test DM performance cases)
2017.11 Start-up / Scale up Selection
2017.11 CDISC Gold Member
2017.12 Completion of International intellectual property right-related dispute Prevention Consulting Business
2017.12 Venture business authorization
2017.07~ 2018.01 Clinical trial performance (Seven Bioquivalence Test DM performance cases)
Date of Establishment2017-03-06
Accelerator시너지아이비투자
StageInformation Communication
Date of TIPS Selection2017-08-16
