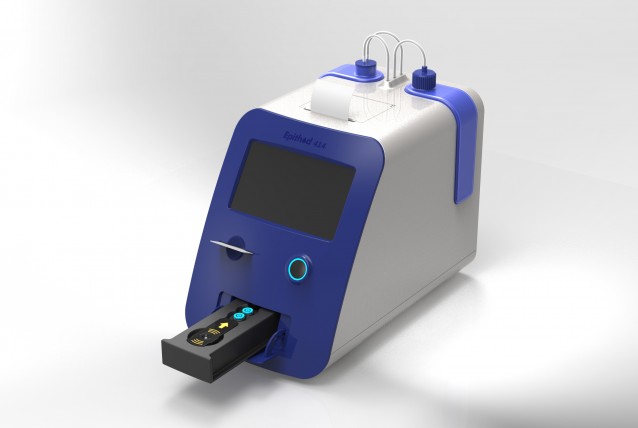


Project
DxGen provides POC products for entire stage of diabetes care from initial assessment to post-diagnosis treatment. Our goal is to help make clinical decisions in the care and treatment of patients at the time and place. Epithod®616 (Ep.616) is the 1st POC analyzer to measure both Glycated Albumin and HbA1c. It is designed to meet laboratory accrediting requirements for in vitro diagnostic. To overcome semi-automated analyzer, we are developing fully automatic analyzer, Epithod®AutoDx (Ep.414). All-in-one 1 step cartridge maximize user convenience and improve marketability, it can consolidate our position as a company specialized in POC.
Project Summary
Epithod®616 is the first POC system for Glycated Albumin and HbA1c, it consists of analyzer and test kits. Test Kit is a new application of the boronate affinity principle utilizing nanoencapsulated color-dyed boronic acid conjugate. The advanced nano-bio technology ensures thermal stabilization of reagents for enhanced consistency and accuracy, it also leads to reduced dependency on cold-chain system.
Furthermore, we acquire technologies for the development of automatic analyzer satisfying usability and cost-effectiveness at the same time and minimizing error from user. Our products can provide simplicity with small volume of blood sample and same-visit results with lab quality and precision.
Furthermore, we acquire technologies for the development of automatic analyzer satisfying usability and cost-effectiveness at the same time and minimizing error from user. Our products can provide simplicity with small volume of blood sample and same-visit results with lab quality and precision.
Business Model
DxGen builds a strong foundation by supplying the analyzer and produce profits by sustainable sales of test kit. Founders and leadership of the company are an experienced team composed of industry leaders with successful track records. We are building a global investor, strategic partner and customer base and plan to manufacture components in multiple locations worldwide. Through providing continuous training (distributor training), we expand marketing reach, widen sales, and make effective the distribution of products and services.
Main Achievements
We, Dxgen, obtain Korea medical devices manufacturing license and a certificate of GMP to ensure that it is appropriate for the medical device and risk involved. Epithod®616 analyzer registered as medical spectrophotometer has been found to comply with Electromagnetic compatibility and safety requirement (EN 61010-1:2010 | IEC 61010-2-101:2015 | EN 61326-1:2013 | EN 61326-2-6:2013) Furthermore, test kit is received a certificate of traceability to the Diabetes Control and Complications Trial (DCCT) Reference Method from NGSP.
We, DxGen, declare under our sole responsibility that the Epithod®616 measuring system with CE mark meet essential requirements, the Council Directive 98/79/EC Annex III concerning medical devices. The Quality Management System is certified under EN ISO 13485:2012 for the design, development, production and distribution of IVD.
We, DxGen, declare under our sole responsibility that the Epithod®616 measuring system with CE mark meet essential requirements, the Council Directive 98/79/EC Annex III concerning medical devices. The Quality Management System is certified under EN ISO 13485:2012 for the design, development, production and distribution of IVD.
Date of Establishment2016-06-16
AcceleratorBNH Investment
StageBiomedical
Date of TIPS Selection2017-06-01
